These days hydrogen is in evidence as an alternative for clean fuel. This publicity is increasing as the hydrogen fuel gets real in transportation.
Although the combustion of hydrogen and oxygen produces only water 2H2 (g) + O2 (g) → 2H2O (g) + energy, what many people do not know is that the main process to produce hydrogen is from fossil fuels by steam reforming or partial oxidation of methane and coal gasification. These processes represent around 95% of hydrogen production.
Hydrogen is applied in many industries, like Metallurgical and steel industry, Petrochemical and refining industry, Glass and float glass manufacturing, Chemical and pharmaceutical industry, Production of H2O2, Food industry, Electronics industry, Technical gases.
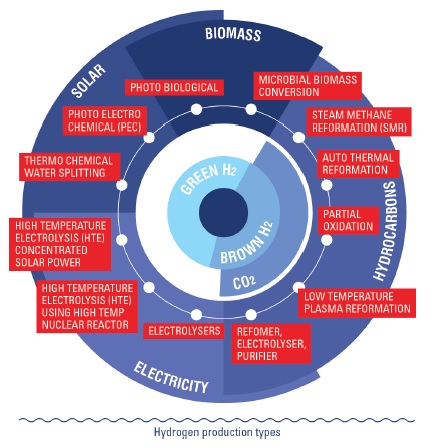
Source: hydroville.be
I have been working with hydrogen production for some years and it is a very interesting process. Its biggest challenges involve sulphur contaminants removal, the good operation of the furnace and the correct tunning of the PSA (pressure swing adsorption) vessels.
PROCESS DESCRIPTION
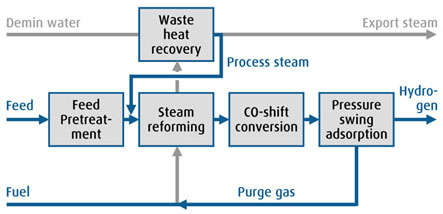
Natural gas is compressed and pass-through some energy recovery in order to increase plant energy efficiency. Before entering the furnace, natural gas is mixed with superheated steam. Carbon-steam ration is controlled to prevent coking formation in the furnace. Hydrogen production happens in two steps, in the first one steam and methane produces syngas with the addition of energy to the reaction. In the second step, the syngas got to the shift reactor where the syngas reacts with steam and produces hydrogen with the release of energy.
The hot stream containing hydrogen is cooled down and goes to the PSA system where the hydrogen is purified removing CO2 and other contaminants. This way, it is possible to have hydrogen purity above 99.999% in volume.
To simulate hydrogen production in Aspen Hysys you need to know the reaction involved in the process and how to set them in the software.
Furnace
For this process, high temperature (700–1100 °C) steam (H2O) reacts with methane (CH4) in an endothermic reaction to yield syngas.
- CH4 + H2O → CO + 3 H2
Shift Reactor
In a second stage, additional hydrogen is generated through the lower-temperature, exothermic, water gas shift reaction, performed at about 360 °C:
- CO + H2O → CO2 + H2
PROCESS FLOW DIAGRAM
The process flow diagram chances depending on the technology licensor. In any case, it is usual to have compressors, heat exchangers, reactor, vessel separator, furnace, pumps etc. The utilities include instrument air, nitrogen, demin water and power.
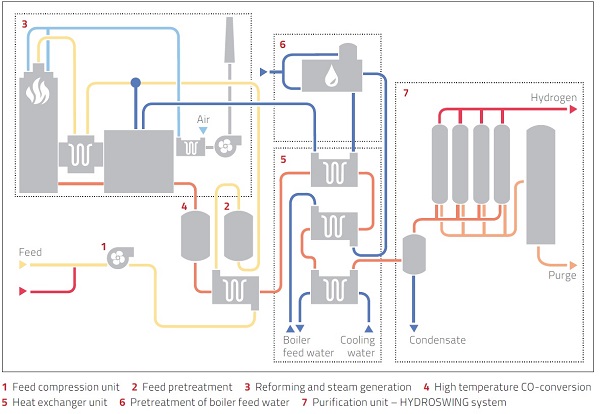
Source: Mahle Advance Gas Systems
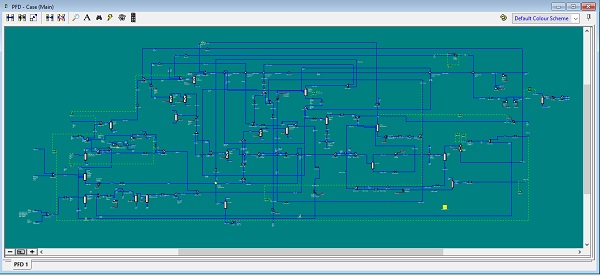
Below you can see how big can be a hydrogen plant process simulation with steam methane reformer.
ADDING EQUILIBRIUM REACTOR IN ASPEN HYSYS SIMULATION
In the video below, besides the overview of a steam methane reforming process, you will learn how to add and set an equilibrium reactor on Aspen HYSYS and Unisim Design.
You can download the block flow diagram used in the video from my Telegram Free Mentoring Channel INPROCESS.
To have more content about chemical process engineering and plant design connect with me and subscribe to my newsletter.
Website: https://www.jefersoncosta.com
Telegram INPROCESS: https://www.jefersoncosta.com/inprocess
My Blog: https://www.jefersoncosta.com/blog
Linkedin: https://www.linkedin.com/in/jeferson-costa-process-engineer




What is kinetics of reaction involved in shift reaction?
What if shift reactor inlet stream inlet temperature sudden increase, what will effect on shift reactor o/l temp?
Hi Savan,
Shift reaction is an exothermic reaction, if you increase temperature it will not benefit the production of hydrogen.
About your first question, I did not get what you want to know, sorry. Could you clarify?
Ótimas informações Jeferson, obrigado por compartilhar!!
Thanks Iury 🙂